This page highlights some current areas of research of our group. While this list is far from comprehensive, we hope it gives the reader an overview of the many types of projects and approaches our group develops. Our lab is primarily focused on the use and development of two solid-state techniques: mechanochemical milling, and accelerated ageing.
Mechanochemical Techniques
Neat and Liquid Assisted Grinding
For neat grinding, solid reactants are placed in a milling jar with balls and milled at a fixed milling frequency for varying amounts of time. Similarly, liquid assisted grinding (LAG) and ion- and liquid assisted grinding (ILAG) follow the same procedure with the addition of grinding additives, i.e. liquid additive and liquid additive in combination with a catalytic salt respectively, used for increased reactivity and activation.
RAging
RAging describes the iterative alternation of short milling with a subsequent accelerated aging process, which is used in enzymatic mechanochemical reactions. This method has among others enabled the degradation of biomass under mild conditions, leading to qualitative yields without the requirement of bulk solvents.
Accelerated Ageing
Accelerated ageing, which operates by first mixing our reactants in our ball mill for a short amount of time, and subsequently exposing these homogenized but unreacted samples to humidity (varies from 70% to 100% depending on the climate chamber). Quantitative conversions have been observed within hours but generally take a few days to weeks.
Resonant Acoustic Mixing
Resonant acoustic mixing (RAM) is one of the non-conventional methodologies, that aims to achieve synthetic procedures that are cleaner, safer as well as more energy- and materials-efficient. It is a novel mechanochemical method that permits chemical transformations on milligram, as well as multi-gram scales, without introducing major changes in equipment design. RAM is a mixer that works through resonant vibration to produce a fast motion. This motion is translated into intensive local mixing zones, therefore, it can homogeneously mix solid-solid, solid-liquid and liquid-liquid materials. In practical, a simple plastic vial is placed on top of a linked base of springs, in which the frequency of the whole base is fixed (60 Hz) but the oscillations are controllable (the acceleration herein and depict in units of g). Unlike, the applications of traditional mechanochemistry are hindered by difficulties of reaction scale-up, as laboratory-scale ball milling processes are not readily adaptable to manufacturing-scale planetary or attrition mills.
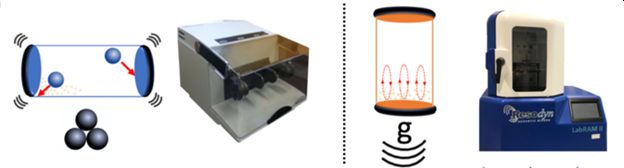
Latest Projects
MOFs and ZIFs
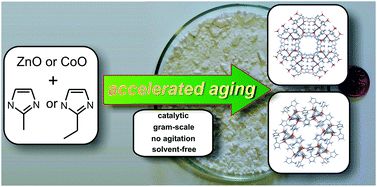
One of the most prominent research areas of our group are Metal Organic Frameworks. Our focus has largely been on developing new cost-effective and environmentally benign syntheses of MOFs and Zeolitic Imidizolate Frameworks using mechanochemistry and our novel "accelerated ageing" technique. One of our first papers on accelerated ageing describes the synthesis of ZIF-8 a highly porous MOF. We have also synthesized a new class of imidazolium frameworks where imidazolium ions bridge anions to create hydrogen bonded frameworks analagous to ZIFs. Additionally, we examine the properties of these materials in regards to their stability towards carbon dioxide.
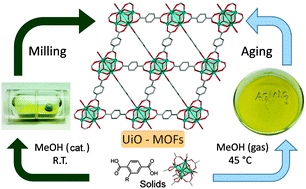
We are currently developing large scale sustainable syntheses of MOFs with interesting properties such as MOF-74 and UiO-66. Our interest in MOFs is not limited to synthetic methodologies and we are synthesizing new MOFs, including the rare organic minerals stepanovite and zhemchuzhnikovite which are porous magnetic and proton conducting metal oxalate MOFs. We continue to synthesize a wide variety of MOF materials and explore their properties for gas storage, catalysis, and fluorescence.
Thermodynamic Stability of MOFs
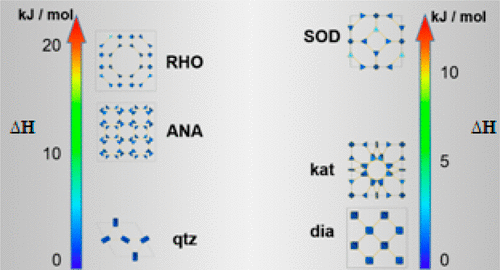
In collaboration with Prof. Alexandra Navrotsky's group at Arizona State University, we have combined solution calorimetry and periodic DFT to analyze the thermodynamics of two families of topologically distinct polymorphs of zinc zeolitic imidazolate frameworks (ZIFs) based on 2-methyl- and 2-ethylimidazolate linkers. These results demonstrate a correlation between density and thermodynamic stability, and allow us to probe the effects of ligand substitution. These results suggest that mechanochemical synthesis of ZIFs proceeds through thermodynamically increasingly stable and more dense phases.
Organometallics
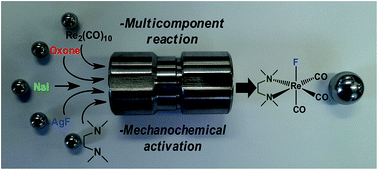
One of our main goals includes the development of mechanochemistry for organometallic synthesis, to conduct oxidative addition, one of the fundamental reaction types of organometallic chemistry. Mechanochemical reactions have recently demonstrated impressive potential for improving the speed, energy- and materials-efficiency of chemical reactions, and especially so in organic chemistry, metal-organic synthesis and syntheses of molecular solids. However, the potential of mechanochemistry in organometallic synthesis has remained almost completely unexplored. In addition to the rhenium complexes below we are investigating both ruthenium based olefin metathesis. Recently we describe the first application of mechanochemistry to conduct a fundamental transformation of organometallic chemistry: oxidative addition.1 By using simple organometallic rhenium(I) precursors as model compounds, we have demonstrated for the direct oxidative addition of halogens (Cl, Br and I) onto a metal centre via a mechanochemical procedure that, unlike all relevant solution procedures reported in almost 50 years, does not require solvent, elementary halogens, or photochemical and high-pressure treatment. Instead, the mechanochemical procedure utilises a conventional oxidation agent in combination with readily available metal halides.
Solvent – free metal separation and recycling
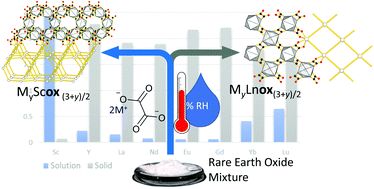
Metals, such as gold, platinum, palladium, etc, are omnipresent in our everyday life and technology. Most of these metals are not phase pure in nature, but occur as mixed ores; mostly oxides, silicates, and sulfides. Their separation, purification, as well as recycling is not only very expensive but also requires the use of hot, concentrated acids, bulk solvent, and a tremendous amount of water. This puts an immense strain on our environment. As green chemist, it is our believe that we can make a difference by developing solvent-free, solid-state separation and recycling of metals from ore-like matrices and metal-waste using cheap, ecologically friendly organic ligands. These reactions are facilitated by mechanochemical grinding and accelerated ageing at room, or slightly elevated temperatures. Therefore, we not only make the process more eco-friendly by avoiding corrosive and hazardous chemicals, generate neglectable solvent waste, and using less energy, but also through recycling of noble metals. In addition, we also have the advantage to directly functionalize the metals during recycling and separation process. We are currently applying our methods towards more efficient lanthanide separations.
Hypergolic Materials
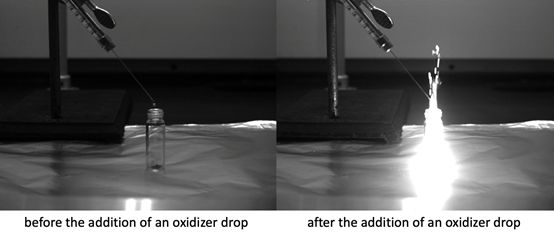
Our group has recently started working on hypergolic materials, i.e. materials that are capable of spontaneous ignition upon contact with an external oxidizer. Hypergolic materials are of critical importance as fuels and propellants in aerospace applications (e.g., rockets and spacecraft). While typical propellants are energetic hydrazine-based molecules that (extremely toxic and cancerogenic!), we synthesized hypergolic MOFs and cocrystals with high combustion energies using solution, and mechanochemical based syntheses. Our approached is based on introducing alkene or alkyne “trigger” groups onto the azolate linkers in popular zeolitic imidazolate framework (ZIF) class of MOFs. Through crystal engineering and material optimizations enable access to high combustion energies and ultrafast ignition delays as low as a 1-2 ms.
Mechanistic Studies of Mechanochemical Reactions
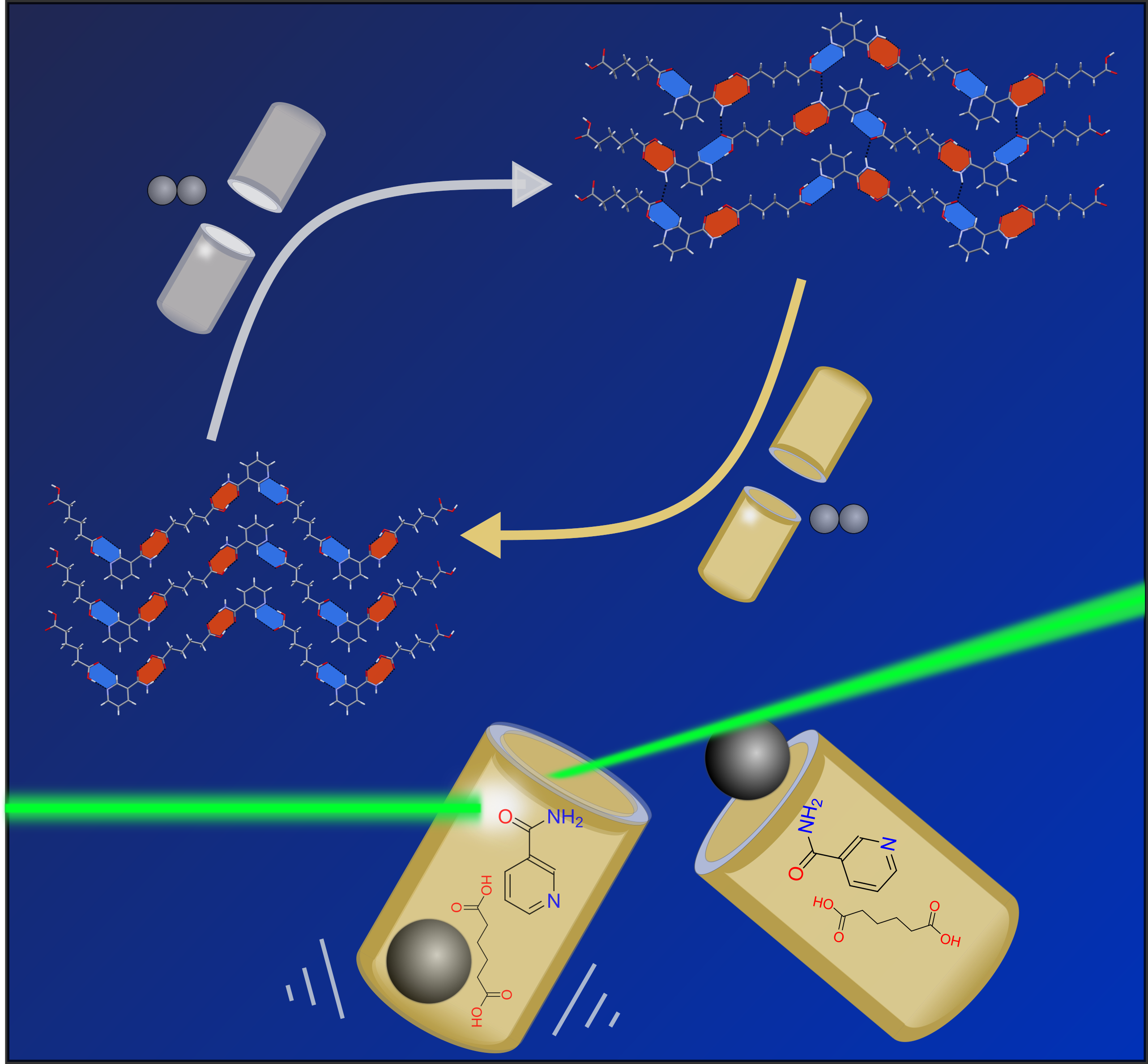
Our group is interested in a better fundamental understanding of underlying reaction mechanism of mechanochemical reactions. In collaboration with international research teams, we pioneered real-time in situ monitoring first by X-ray powder diffraction. This work has originally been performed at the European Synchrotron Radiation Facility (ESRF), nowadays spread over to other European synchrotron, such as BESSY, SLS, and DESY. Currently, we utilize time-resolved X-ray powder diffraction and Raman spectroscopy to not only monitor mechanochemical reactions of MOFs, ZIFs, cocrystals, and other materials in order to elucidate their reaction mechanism and kinetics, but also to obtain a better understanding of the individual reaction parameters, such as milling frequency, grinding additives, milling assembly, etc. These techniques have enabled us to make ground-breaking discoveries, such as observation of unknown metastable phases and ZIF frameworks with a previously unknown topologies.
Medicinal Mechanochemistry
Our research interest surrounds the synthesis
of biologically active and pharmaceutically relevant organic
compounds via the use of mechanochemistry and sonochemistry,
as an alternative route that is more environmentally benign
when compared to traditional methodologies.
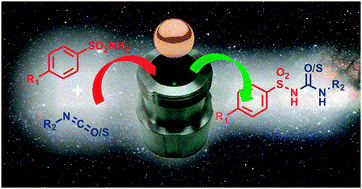
Thioureas
We have recently developed a greener and more highly efficient
protocol for the direct synthesis of unsymmetrical thioureas
and sulfonyl-(thio)urea compounds via a click mechanochemical
coupling of the respective amines, sulfonamides and
(thio)isocyanates without the use of bulk organic solvents.
While inherently poor nucleophilicity of the sulfonamide
nitrogen atom reduces its reactivity in addition reactions, we
reported the use of potassium carbonate as a mild,
environmentally benign base for the deprotonation of the
sulphonamide group as well as a catalytic coupling between the
sulphonamide and (thio)isocyanate via the use of
substoichiometric amounts of CuCl. Furthermore, a second
generation anti-diabetic sulfonyl-urea drug, Glibenclamide,
has also been successfully synthesized. We are expanding this
methodology to other synthetic reactions.
Catalytic Reactions
Our lab has pioneered the development of mechanochemical olefin metathesis reactions using commercially available ruthenium complexes. This rapid, room-temperature approach enables us to minimize the amount of solvents needed in these high-yielding reactions.
Chemical Education

Our group is strongly committed to improving the quality of chemistry education at the undergraduate level by designing new experiments for our teaching labs. Currently we are focused on generating modern experiments involving mechanochemistry for introductory undergraduate courses. We are developing new green chemistry experiments that enable students to evaluate the cost and sustainability of various synthetic methods. In addition, we are focusing efforts on generating new conceptual frameworks for designing upper year teaching labs in order to better integrate important concepts in chemistry into experiments that highlight current challenges in research and teach fundamental lab techniques.
Collaborative Work
Mechanoenzymatic Reactions with Auclair Group
In collaboration with the Auclair Group we are developing mechanoenzymatic reactions to, amongst others, reduce and recycle polymers, such as PET, as well as naturally abundant biopolymers including chitin and cellulose.
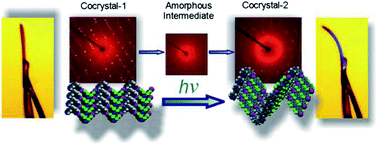
Azobenzenes with Barrett Group
In a collaboration with the Barrett group, perhalogenated cis-azobenzenes have shown the first photomechanical isomerization which permanently modifies crystal shape. This thermally irreversible change involves a large change in crystal shape, and importantly is controllable. It is the first time an irreversible photomechanical change in crystal shape has been reported in azobenzenes.
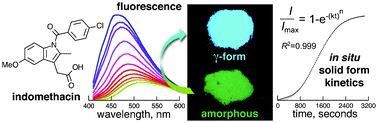
Fluorescence with Cosa Group
Working with members of the Cosa group who specialize in fluorescence, we have use solid state fluorescence to monitor and characterize polymorphs, solvates, and cocrystals of pharmaceutical molecules. This provides our group with the important ability to quantify the amounts of amorphous and crystalline phases as well as monitor kinetics of interconversion. We hope to expand this technique to other types of solid material as well as mechanochemical processes.
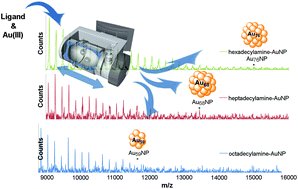
Nanoparticles with Moores Group
In collaboration with the Moores Group we have recently investigated the syntheses of ultra-small nanoparticles using our ball milling technique. We report gram scale syntheses of gold nanoparticles with diameters between 1-4 nm, and bismuth sulfide nanoparticles with diameters around 2nm. The significance of this work is that we manage to avoid the use of external reducing agents or bulk solvents which are typically required for nanoparticle synthesis. We believe that this presents a new scalable, cost effective, and environmentally benign synthesis of nanoparticles.
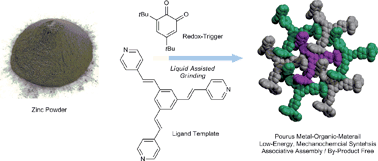
Redox Driven Self-Assembly with the Lumb Group
Our collaboration with the Lumb Group is focused on combining solid state oxidations and coordination driven self-assembly to achieve the nearly waste-free synthesis of novel metal organic materials with magnetic properties. Additionally, such protocols can be used to create germanium based advanced materials and synthetic precursors, without the need for chlorine.